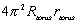17. Life Support Systems
The Life Support Systems on DL4
were chosen in an attempt to close the system loop as much as possible. This
was achieved by choosing subsystems that produced by-products that were compatible
with the inputs of the surrounding systems. The most important, and hardest
to control, aspects of the life support system are its biological components,
including humans, agricultural plant life, recreational plant life, biological
nitrification and biological waste decomposition. All figures for the life
support systems are based on the approximate input and output rates of the first
three of these. This allows other more technological systems to be controlled
depending on the conditions at that time.
The overall system was divided into thirteen main
subsystems. These were:
1. Urine Processing
2. Waste Processing
3. Nitrogen Makeup
4. Temperature Control
5. Humidity Control
6. Water Monitoring
7. Water Processing
8. Oxygen Generation
9. Carbon Dioxide Concentration
10. Carbon Dioxide Removal
11. Trace Contaminant Control
12. Particulate Removal
13. Atmospheric Monitoring
17.1 Urine Processing
Urine is 95% water (ref. 24), the rest being
made up of more than 100 various organic and inorganic substances. These include
urea (13-20g/L), sodium chloride (8-12g/L) and various acids (up to 3g/L).
All these contaminants must find their way back into the loop in order to maintain
a closed system. Options considered to attain this included Biological Decomposition,
TIMES (Thermoelectric Integrated Membrane Evaporation System), AES (Air Evaporation
System), VCD (Vapour Compression Distillation), and VAPCAR (Vapour Phase Catalytic
Ammonia Removal).
Although all systems had their
advantages and disadvantages VAPCAR was considered to be most capable of producing
high quality water and operating with little or no need for re-supply. The
other options were ruled out for a number of reasons: Biological Decomposition
required the growth of micro-flora at 60°C, giving rise to fears of bacterial epidemics and denitrification;
TIMES required pre-treatment with ozone, which is unstable and hazardous, and
its small Nafion tubes could easily be blocked; AES, although providing 100%
water recovery, periodically required replacement wicks, produced a foul smell
and did not return the 5% contaminant portion of urine to the system; and finally,
VCD, although coming a close second, still required pre- and post-treatment,
and its water quality varied depending on the number of organic compounds that
co-condensed with the water.
VAPCAR is based on a catalytic
chemical process, which eliminates the need for expendable chemicals and oxidises
impurities to innocuous gases that can be used to replace the settlement’s atmosphere.
Fig. 17.1a shows a simplified flow diagram of the VAPCAR
process. Urine is initially fed through the evaporator, which consists of a
bundle of hollow fibre membranes made from perflourinated ion-exchange polymer.
Here the urine passes through the interior of the fibres and is vaporised through
their exterior. Next the feed vapour is put through the first catalyst bed.
This consists of 0.5% platinum on alumina oxidation catalyst pellets and operates
at about 523K. The bed oxidises ammonia to nitrous oxide (N2O) and
nitrogen while volatile hydrocarbons are oxidised to carbon dioxide and water.
This bed then leads to a condenser-separator where the freshly processed water
is removed from the system. The remaining feed is passed through the second
catalyst bed, consisting of 0.5% ruthenium on alumina catalyst pellets, which
operates at 723K. Here the nitrous oxide is decomposed to nitrogen and oxygen,
which can be used to replenish the settlement’s atmosphere. The remaining recycle
and vapour loops in the system are maintained above pasteurisation temperature
(347K), using wet heat, in order to prevent microbial growth and thus increase
water quality (ref. 18).

 VAPCAR produces
near potable water, which only requires slight alteration of its pH to meet
the necessary standards (detailed later in 17.5 Water Monitoring). Therefore,
the water produced can either be put into plant circulation for further purification
or placed after being tested in potable water storage for human consumption,
depending on the needs at the time (see fig. 17.12a). The remaining
outputs are mostly gases that can go directly into the atmosphere. Some problems
with the compressor system within VAPCAR have arisen, however these should be
solved shortly and given the futuristic nature of this project are not seen
as posing any significant difficulties.
VAPCAR produces
near potable water, which only requires slight alteration of its pH to meet
the necessary standards (detailed later in 17.5 Water Monitoring). Therefore,
the water produced can either be put into plant circulation for further purification
or placed after being tested in potable water storage for human consumption,
depending on the needs at the time (see fig. 17.12a). The remaining
outputs are mostly gases that can go directly into the atmosphere. Some problems
with the compressor system within VAPCAR have arisen, however these should be
solved shortly and given the futuristic nature of this project are not seen
as posing any significant difficulties.
17.2
Waste Processing
Waste products within a life support system mainly
consist of faeces and food preparation wastes. Due to the use of plants as
a food source on board DL4, a large amount of inedible plant material will have
to be catered for in order to return the carbon to the plants. Inedible plant
material will take up 98% of the total dry solid waste. The remaining waste
will be produced in semi-solid, solid or mixed forms from humans and from concentrates
from the atmosphere and water purification systems.
For the large percentage of inedible plant material
that must be dealt with aerobic biological decomposition (mainly composting)
was chosen. This produces liquid and gas effluents that are, for the most part,
compatible with plant growth and less toxic than the effluents produced by alternative
physio-chemical systems. The system’s production of nitrates instead of ammonia
reduces denitrification, although some nitrification will be necessary for any
ammonia produced and for the phytotoxic NO2.
Anaerobic decomposition was dismissed on the basis
that it produces methane, which, although capable of being used as a fuel, is
incompatible with any of the other life support subsystems. Aerobic decomposition
does still produce some difficulties however. Like all biological systems it
is difficult to control with slow start up and turnover times and the risk of
producing epidemic micro-organism populations. These problems can be overcome
by altering the bioreactor’s flux rate, temperature, pH levels and the incoming
waste composition and thus controlling the organisms’ equilibrium. Even if
difficulties arise in the bioreactor it is easily repaired due to its relative
simplicity.
Another system for waste treatment will also be
in place to deal with more toxic materials, which are less compatible with biological
decomposition. For instance, human faeces, which although easily subjected
to biological decomposition, produce a foul smell and its decay is difficult
to control safely. Therefore, physio-chemical systems, such as: Incineration,
SAC (Starved Air Combustion), the RITE (Radio Isotope Thermal Energy) Process,
Wet Oxidation, and SCWO (Super Critical Wet Oxidation) were considered.
SAC, although using less oxygen than complete incineration
was dismissed as it produces toxic fumes; the RITE process, although utilising
the safer catalytic oxidation method, also used a plutonium heat source making
its servicing a risk on such a long term project; Wet Oxidation required a detailed
knowledge of the input material in order to predict the compounds produced,
while SCWO was chosen as it is capable of handling all settlement waste, including
the removal of trace contaminants directly from the process atmosphere. This
ability to handle all spacecraft waste offers a useful alternative to VAPCAR
should any difficulties arise with the urine processing subsystem.
SCWO makes use of water in its super critical state
(above 647K and 2.21 x 107Pa) as a medium for the oxidation of usually
insoluble compounds. These insoluble compounds become soluble in super critical
water (including O2), allowing the oxidation of all wastes in a single
phase, assuming sufficient oxygen is available in the process atmosphere and
the reactor is above 922K and 2.53 x 107Pa. The temperature allows
for extremely short reaction times with complete oxidation of organic compounds,
including trace contaminants, to carbon dioxide, hydrogen, and nitrogen, in
less than one minute. With proper design the heat generated by oxidation can
be used to maintain the reactor temperature thus requiring little external energy
(ref. 18).
As in VAPCAR, the SCWO process produces a potable
water supply, which can then be either further purified or sent to potable water
storage for human consumption. Some difficulties have been noted with reactor
material corrosion, leading to contamination of the outputs, however, again
as in VAPCAR, these problems are not seen as long term dilemmas.
All the details above regarding Waste Processing
deal specifically with Life Support Systems. Additional subsystems may have
to be implemented for specific wastes produced under unusual conditions, i.e.
– lab work (see Trace Contaminant Control).
17.3
Nitrogen Makeup
Nitrogen will be necessary in the atmosphere as
an inert gas, in order to make up the necessary pressure within the settlement.
Apart from this, nitrogen will be necessary for plant growth as it forms part
of their protein makeup. Although it is necessary for plants it is poisonous
to humans, if metabolised, and most amino acids consumed undergo deamination
in the liver. This process involves the removal of nitrogen from the molecules
and then its excretion as urea in urine. Thus the return of human consumed
nitrogen to the atmosphere is achieved through the VAPCAR process (detailed
earlier). Some more lost nitrogen is returned to the atmosphere through SCWO
while the rest is contained within the inedible plant material and food processing
wastes. This is not returned directly to the atmosphere but instead, through
biological decomposition, takes the form of nitrous oxides and a little ammonia
(see fig. 17.12a).
These compounds can be fed through a biological
system of Nitrification, which would convert Ammonia (NH3) to Nitrite
(NO2) through bacterial oxidation (i.e. – by Nitrosammonas),
and Nitrite to Nitrate (NO3) again through bacteria (i.e. – Nitrobacter).
Nitrate (some of which is produced directly by biological decomposition of wastes)
is usable by plants and readily converted to plant proteins, nitrites however
are phytotoxic and must go through this process. If this process does not produce
enough nitrogen for plant usage, nitrogen fixation can take place directly from
atmospheric nitrogen by symbiotic bacteria such as Rhizobium in conjunction
with legume crops. On the other hand, if too many nitrates are produced, resulting
in a loss of atmospheric nitrogen, denitrification of nitrates can take place
through bacteria such as T. Denitrificans. This safety feature can be
set up as a negative feedback loop as the level of atmospheric N2
rises or falls. All these biological systems can be controlled as with the
biological decomposition of wastes, through the alteration of the organisms’
equilibrium.
Although the above systems should recycle all nitrogen
and close the loop, any loss of atmosphere through leakage would be most noticeable
in nitrogen, due to its relatively high partial pressure in the atmosphere.
However, apart from trying to prevent leakage, such losses cannot be made up
from resources onboard the settlement, therefore some re-supply may be required
periodically, possibly from a cryogenically stored supply or from future discoveries
of asteroid sources.
17.4
Temperature and
Humidity Control
Due to DaedalusaL4’s’ location at L4, providing
sunlight 99% of the time, direct solar heating should provide sufficient heat
for the human habitat. However this will still require control to prevent overheating
and to transfer the heat to those areas that will receive the minimum of direct
solar heating. This can be achieved by using a CHX (Condensing Heat Exchanger).
Luckily this system can also remove moisture from the atmosphere extending its
capability into humidity control.
The temperature must be maintained at a suitable ‘shirt
sleeve’ temperature (20-25°C) to give a comfortable working
and living environment in the human habitat. This temperature will have to
be varied for different areas of the settlement such as laboratories, agricultural
areas and industrial areas. However, the humidity must also be maintained at
a suitable level for human habitation (about 40%), to allow the transpiration
of agricultural plants, and to prevent the condensation of water on instruments.
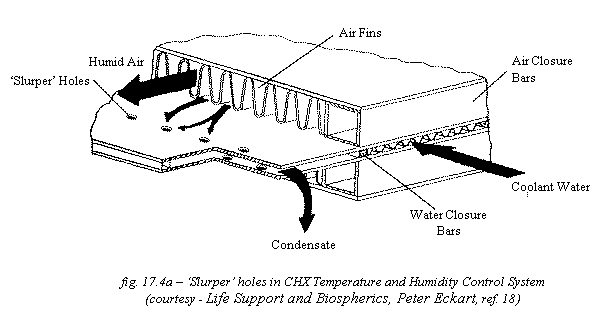
First, in a CHX, the air temperature is lowered
to below dew point by passing it over an enclosed coolant water loop. This
water loop can then be used to transfer the collected heat to other areas of
the settlement that require it. Meanwhile, the air is cool enough so that a
condensate film forms from the water-laden air. This film of water is then
removed from the subsystem through small holes called ‘slurper holes’ (see fig.
17.4a). The main stream of air continues on back to the atmosphere, while
liquid water is precipitated out of the condensate. This subsystem can be used
to remove transpiration water from the atmosphere surrounding plants and thus
produces a potable water supply. Varying either the number or size of the slurper
holes can control the process. It will probably prove easier to vary the number
of holes, closing off some when the humidity is low enough.
Other options considered for humidity control included:
Molecular Sieves, Membrane Separation and Water Vapour Electrolysis. Molecular
Sieves and Membrane Separation were dismissed due to their complex fine design
compared to the technological simplicity of CHX. Water Vapour Electrolysis
offered no advantages in terms of humidity control over CHX apart from its small
size, however it was considered as a backup portable emergency oxygen generation
unit and as such would also function as a humidity control device.
Using CHX, the amount of heat energy trapped on DL4 can be regulated and transferred
between different sections of the settlement. However, sufficient heat energy
must initially be provided to maintain the shirt sleeve climate before the CHX
can transfer it. In order to calculate the amount of heat energy required,
the power balance of the station must be found.
A power balance is influenced
by the energy per unit time created within the object, energy per unit time
coming from outside and energy per unit time given to outside. Within our solar
system, the sun is the only major source of energy outside, however to attain
a power balance the planetary reflected solar input has to be taken into account.
Thus the power balance (ref. 18)
becomes:
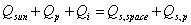
where,
solar input to spacecraft: 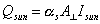
planetary-reflected solar input: 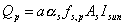
internally generated power: 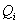
power radiated from spacecraft to space: 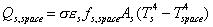
power radiated from spacecraft to planet: 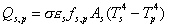
planetary albedo: 
spacecraft surface absorptivity: 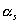
spacecraft surface emissivity: 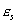
view factor spacecraft-to-space: 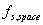
temperature of the spacecraft: 
space background temperature: 
solar energy flux: 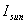 = 1,390W/m2
= 1,390W/m2
area of spacecraft: 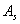
projected area of the spacecraft: 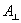
view factor spacecraft-to-planet: 
temperature of the planet: 
Stefan Boltzmann constant: 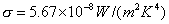
Assuming that 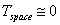 K
(in reality,
K
(in reality,  K)
and
K)
and  ,
in equilibrium condition the energy balance becomes:
,
in equilibrium condition the energy balance becomes:

Located at L4, approximately 384,400km from Earth,
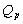 and
and
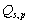 are
negligible and the equation may be simplified to:
are
negligible and the equation may be simplified to:
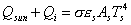
yielding the equilibrium temperature of the spacecraft:
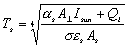
For DL4, this equation can now be used to calculate the temperature of the
torus given a few assumptions. Firstly, it is assumed that the settlement’s
hull is made entirely of aluminium. Therefore, the surface absorptivity,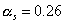 (ref. 44) and, the surface emissivity,
(ref. 44) and, the surface emissivity,  (ref. 80).
Also, taking the approximate internal power output (
(ref. 80).
Also, taking the approximate internal power output (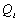 )
of the torus as 69,007.5kW (see below), the surface area[1]
(
)
of the torus as 69,007.5kW (see below), the surface area[1]
(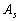 )
as 5,260,499.146m2 and the projected surface area[2]
(
)
as 5,260,499.146m2 and the projected surface area[2]
(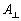 )
as 1,674,349.504m2 and using
)
as 1,674,349.504m2 and using 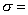 5.67
5.67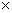 10-8W/(m2K4)
a value for
10-8W/(m2K4)
a value for 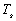 can
be deduced:
can
be deduced:
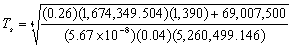
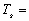 487.5468837K
or @ 214.4°C
487.5468837K
or @ 214.4°C
This value is clearly far above our desired torus
‘shirt sleeve’ temperature and so must be reduced drastically. This has been
catered for to a certain by the thermal radiators mounted on the central sphere,
however at the above temperature, the water coolant of the CHX will vaporise
and thus be useless at transferring heat to the radiator. Therefore, additional
design features must be considered.
Firstly, it must be remembered that the hull is
not made entirely from aluminium, it is, in fact, covered in meteorite shielding,
made of windows and, in places, surrounded by a sheath of radiation shielding.
Therefore, if it is assumed that the majority of the hull is coated in Mylar,
Kevlar and foam, a slightly more suitable equilibrium temperature can be deduced,
with one slight alteration. The addition of a white ‘beta-cloth’, Teflon coated
fibreglass, layer to the settlement adjusts the values of 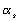 and
and
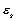 to
0.05 and 0.5 respectively. This changes
to
0.05 and 0.5 respectively. This changes 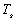 dramatically,
yielding:
dramatically,
yielding:

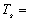 181.573K
or @ –91.6°C
181.573K
or @ –91.6°C
Once again, this is clearly not suitable, however now
at least we can more easily adjust the temperature by adding power to the settlement,
transferring heat through the CHX (once sufficient power has been given to liquefy
the coolant), and radiate excess heat through the thermal radiator.
Using the equation:

the amount of extra power required (x) to warm
the settlement can be deduced, assuming 69,007,500W have already been supplied
through everyday activities and the desired temperature (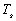 )
is 23°C or 296.15K.
)
is 23°C or 296.15K.

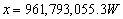
This extra power can easily be provided by electric
heating throughout the torus, however an additional power source has gone unnoticed
in the above calculations.
The central sphere will be devoted to novel industry
and research methods and thus the 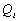 value
for this area is unknown. However, given the high temperature and power requirements
of material refinement and construction, it can be assumed that this value will
be high. Therefore, the independent temperature of the central sphere in equilibrium
is anticipated to exceed the desired shirt-sleeve climate. This means that
such heat could be monitored and transferred through the CHX system to the torus
habitat to increase the temperature within this area, thereby reducing the dependency
of the settlement on purposeful heating (although some will still be required),
while introducing the torus as a heat sink and lowering dependency on the thermal
radiator.
value
for this area is unknown. However, given the high temperature and power requirements
of material refinement and construction, it can be assumed that this value will
be high. Therefore, the independent temperature of the central sphere in equilibrium
is anticipated to exceed the desired shirt-sleeve climate. This means that
such heat could be monitored and transferred through the CHX system to the torus
habitat to increase the temperature within this area, thereby reducing the dependency
of the settlement on purposeful heating (although some will still be required),
while introducing the torus as a heat sink and lowering dependency on the thermal
radiator.
It should however be noted that the value used for
the torus 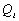 above
is extremely approximate. It was formulated on the basis of a 27,203,000W requirement
for food processing services, a similar figure for torus industry and research,
and an extra home power consumption of 1,360.15W/person. In addition to this
an average value of 100W was taken as the power generation of the average resident.
(This figure was arrived at by taking the average amount of food consumed per
day as 2,000Cal: 2,000Cal/day = 2,000kcal/day = 2,000,000cal/day = 8.37Mjoule/day
= 349kjoule/hour = 97joule/sec = 97W.) Thus the total figure of 69,007,500W
(27,203,000W + 27,203,000W + 13,601,500W + 1,000,000W) was deduced. This value
will change over time, given different industrial activities, the diurnal range
and human metabolic rates. Thus constant monitoring and control of the heat
being transferred by the CHX system will be required, along with careful alignment
of the ‘slurper’ holes to prevent over-dehumidification, given such extensive
use of the system.
above
is extremely approximate. It was formulated on the basis of a 27,203,000W requirement
for food processing services, a similar figure for torus industry and research,
and an extra home power consumption of 1,360.15W/person. In addition to this
an average value of 100W was taken as the power generation of the average resident.
(This figure was arrived at by taking the average amount of food consumed per
day as 2,000Cal: 2,000Cal/day = 2,000kcal/day = 2,000,000cal/day = 8.37Mjoule/day
= 349kjoule/hour = 97joule/sec = 97W.) Thus the total figure of 69,007,500W
(27,203,000W + 27,203,000W + 13,601,500W + 1,000,000W) was deduced. This value
will change over time, given different industrial activities, the diurnal range
and human metabolic rates. Thus constant monitoring and control of the heat
being transferred by the CHX system will be required, along with careful alignment
of the ‘slurper’ holes to prevent over-dehumidification, given such extensive
use of the system.
17.5 Water Monitoring
Water Monitoring must take place to ensure that all
potable and hygiene water satisfies the following physical parameters (table
17.5a), and maintains safe levels of chemical and biological contaminants
(table 17.5b):
|
Physical Parameters
|
Limits (Potable Water)
|
Limits (Hygiene Water)
|
|
Total Solids (mg/L)
|
100
|
500
|
|
Colour True (Pt/Co units)
|
15
|
15
|
|
Taste (TTN)
|
3
|
3
|
|
Odour (TON)
|
3
|
3
|
|
Particulates
(max. size in microns)
|
40
|
40
|
|
pH
|
6 – 8.4
|
5 – 8.4
|
|
Turbidity (NTU)
|
1
|
1
|
table 17.5a - Physical parameters for potable and hygiene
water (ref. 18)
|
Inorganic Constituent
|
Limit (mg/L)
|
|
Ammonia
|
0.5
|
|
Arsenic
|
0.01
|
|
Barium
|
1
|
|
Cadmium
|
0.005
|
|
Calcium
|
30
|
|
Chloride
|
200
|
|
Chromium
|
0.05
|
|
Copper
|
1
|
|
Iodine
|
15
|
|
Iron
|
0.3
|
|
Lead
|
0.05
|
|
Magnesium
|
50
|
|
Manganese
|
0.05
|
|
Mercury
|
0.002
|
|
Nickel
|
0.05
|
|
Nitrate (NO3-N)
|
10
|
|
Potassium
|
340
|
|
Selenium
|
0.01
|
|
Silver
|
0.05
|
|
Sulphate
|
250
|
|
Sulphide
|
0.05
|
|
Zinc
|
5
|
|
|
Bactericide
|
Limit (mg/L)
|
|
Residual Iodine
|
0.5 – 6
|
|
|
Aesthetics
|
Limit (mg/L)
|
|
Cations
|
30
|
|
Anions
|
30
|
|
Carbon Dioxide
|
15
|
|
|
Bacteria
|
Limit (CFU/100ml)
|
|
Total count
|
1
|
|
Anaerobes
|
1
|
|
Aerobes
|
1
|
|
Gram negative
|
1
|
|
Gram positive
|
1
|
|
Coliform
|
1
|
|
Enteric
|
1
|
|
|
Organic
Parameters
|
Limit (g/L)
|
|
Total acids
|
500
|
|
Cyanide
|
200
|
|
Halogenated Hydrocarbons
|
10
|
|
Phenols
|
1
|
|
Total Alcohols
|
500
|
|
Total Organic Carbon (TOC)
|
500 (Potable Water)
10000 (Hygiene Water)
|
|
Uncharacterised TOC (UTOC)
|
100 (Potable Water)
1000 (Hygiene Water)
|
| |
|
|
Virus (PFU/100ml)
|
1
|
| |
|
|
Yeast and Mould (CFU/100ml)
|
1
|
table 17.5b – Chemical and biological contaminant
limits in potable and hygiene water (ref. 18)
 In
order to ensure these parameters are met, the pH, ammonia content, total organic
carbon (TOC), electrical conductivity and microbial concentration must be frequently
monitored. Microbial content can be monitored indirectly by measuring the iodine
content of the water, thus ensuring the constant presence of a biocide. A suitable
procedure for water quality monitoring is given in fig. 17.5a. This
could easily be implemented before water produced from CHX, VAPCAR and SCWO
subsystems are inputted into water storage.
In
order to ensure these parameters are met, the pH, ammonia content, total organic
carbon (TOC), electrical conductivity and microbial concentration must be frequently
monitored. Microbial content can be monitored indirectly by measuring the iodine
content of the water, thus ensuring the constant presence of a biocide. A suitable
procedure for water quality monitoring is given in fig. 17.5a. This
could easily be implemented before water produced from CHX, VAPCAR and SCWO
subsystems are inputted into water storage.
A decision that is open to opinion is whether or not
to fluoridate drinking water. Fluoridation has been used for many years to help
prevent dental caries. Recently, there has been a change in public opinion.
There are fears that fluorine in drinking water can cause other health risks.
We considered the arguments for and against fluorination from many sources (ref.
86,87,89).
The American Dental Association is still for the fluoridation
of community water supplies. In their 1999 ‘Fluoridation Facts’ report, they
stated:
“Fluoridation is considered beneficial by the overwhelming
majority of the health and scientific communities as well as the general public.
· Fluoride helps prevent tooth decay. All ground
and surface water in the U.S. contains some naturally occurring fluoride. If
a community’s water supply is fluoride-deficient (less than 0.7 parts fluoride
per million parts water) fluoridation simply adjusts the fluoride’s natural
level, bringing it to the level recommended for decay prevention (0.7-1.2 parts
per million).
· Fluoridation is a community health measure
that benefits children and adults. Simply by drinking optimally fluoridated
water, members of a community benefit, regardless of income, education or ethnicity
— not just those with access to dental care.
· Fluoridation protects over 360 million people
in approximately 60 countries world-wide, with over 10,000 communities and 145
million people in the United States alone.
· As with other nutrients, fluoride is safe
and effective when used and consumed properly. From time to time, opponents
of fluoridation have questioned its safety and effectiveness. None of these
charges has ever been substantiated by generally accepted science. After 50
years of research and practical experience, the overwhelming weight of scientific
evidence indicates that fluoridation of community water supplies is both safe
and effective.” (ref. 88)
We found that there was little conclusive evidence
that the low fluoridation levels that are employed to prevent dental caries
pose any significant threat to health. Any health risks evolved from poor control
of the levels of fluorine in the water, and in toothpaste given to children.
We decided to design for the fluoridation of drinking water, with strict controls
and monitoring. If in the meantime, conclusive proof can be found that the
low levels of fluorine used to prevent dental caries have a significant adverse
effect on people’s health, then the design could be adapted accordingly.
17.6 Water Processing
Water processing is necessary in order to supply
the inhabitants with water, usable both as potable and hygiene water. Most
of the current physio-chemical methods for recycling this supply involve filtration,
which leads to a loss of materials and the need for replacement filters (i.e.
- Reverse Osmosis, Multifiltration and Ultrafiltration). However, about 40%
of the potable drinking water needs (about 1.61L/person/day, excluding all water
in food, 1.15L, and food preparation water 0.79L, 0.04L of which is never consumed
due to evaporation) of DL4 have already been catered for in earlier sections
(17.1 Urine Processing and 17.2 Waste Processing) as VAPCAR and SCWO combined
will produce about 0.45L potable water/person/day. These sources only require
testing and under certain circumstances, limited processing, before being ready
for human consumption and thus supply a relatively quick recycling loop for
water. However, this still leaves the remaining drinking water (0.7L/person/day),
all the food preparation water (0.79L/person/day) and hygiene water (0.36L oral
+ 1.81L hand and face wash + 5.44L shower + 12.47L clothes wash + 5.44L dish
wash = 25.52L/person/day, ref. 31) to be catered for. Plants, during
their growth season, also require an additional 0.45L/plant/day in order to
photosynthesise and maintain turger pressure through osmosis (hence, on average
everybody consumes 1.15L of water per day through food). Using the CHX temperature
and humidity control system extensively, plant transpiration water can be taken
from the atmosphere and used as a potable source of water. This source of clean
water will easily cover the remaining needs of DL4, which are not catered for
by SCWO and VAPCAR.
However, a surplus of water will be maintained onboard,
leading in total to one weeks consumable supply being stored. This surplus
will be utilised for industrial purposes (if these processes do not incorporate
water recovery then water will have to be shipped to the settlement along with
the other materials being refined) and will cover the time lag experienced due
to the slow biological nature of the water recycling system. It will also be
an emergency water source and could help with fire suppression or be used for
water electrolysis and the emergency supply of oxygen, for respiration, or hydrogen,
as a fuel. Based on the approximate figures that: 30% of agricultural area
will be taken up with plants capable of growing at a density of up to 2,000
plants/m2, although these will more likely be grown at about 1,300
plants/m2; 40% will be used for vine like plants and legumes growing
at about 750 plants/m2; and the remaining 30% will be used for low
density crops at about 20 plants/m2 (ref. 49), then, given
an agricultural area of 869,056m2, with only 217,300m2
of this being in season, and therefore of real significance for this calculation,
68,047,084L of potable water will be required by the plants per day (leaving
a wide margin for error and for those plants not in season). In addition to
this 284,100L will be required by the inhabitants per day for hygiene and metabolic
processes. Therefore a total of 478,318,288L of potable water will be kept
on DL4 (this does not include recreational water for swimming pools and natural
waterways). On top of this figure a further half-week’s water will be stored
within the waterways and lakes of the recreational areas. This large amount
is necessary to compensate for the inevitable mixing between recreational and
potable water in the atmosphere and allow for the slow recycling loop that will
return such water to the recreational areas (which will no doubt require monitoring
and topping up from potable stores). This extra water will bring the total
stored water onboard to 717,477,432L.
17.7 Oxygen Generation
Oxygen aboard spacecraft is currently ‘scavenged’ from spent fuel tanks and
supplied from a stored cryogenic source, however in order to maintain a CELSS
(Closed Environment Life Support System), it will be necessary to recycle
oxygen used in human respiration. Physio-chemical means of achieving this
involve the electrolysis of water vapour to produce hydrogen and oxygen.
The oxygen then would go into the atmosphere while the hydrogen would be used
to reduce carbon dioxide, thus producing a fresh source of water to undergo
electrolysis. This process takes care of both oxygen generation and carbon
dioxide removal, however leaves carbon out of the cycle at the end, leaving
no other option but its oxidation through SCWO to CO2. Ignoring
the final oxidation of carbon, the process would yield 0.74kg of oxygen per
person per day (based on an average 1kg carbon dioxide produced per person
per day, ref. 18). This does not completely close the loop, as approximately
0.85kg of oxygen are required per person per day (ref.
31), however such a system could be used in emergencies or when large
numbers of the inhabitants congregate in an enclosed area.
Such systems include the use of Water Vapour Electrolysis,
which, as mentioned earlier, performs the second duty of lowering humidity,
but also the more promising Direct Carbon Dioxide Electrolysis. This solid
electrolyte system can electrolyse both carbon dioxide and water vapour directly
from breath, however the simultaneous electrolysis of water leads to the release
of hydrogen into the atmosphere making this an unsafe process for use over long
periods of time (the hydrogen would be removed at a later stage by the trace
contaminant control systems). Initial electrolysis produces oxygen but also
carbon monoxide, which then passes through a catalytic bed that decomposes it
to solid carbon and carbon dioxide. The CO2 then passes through the
electrolyte again. This process is only used as a backup system and thus is
not included in the life support flow diagram (fig. 17.12a), however
hydrogen-carbon dioxide reduction and water vapour electrolysis are, as sinks
for the hydrogen produced through SCWO. The water vapour electrolysis in this
case is also only in use as a backup system in case of oxygen depletion, otherwise
the water produced from the previous CO2 reduction can be stored
in the potable water store (while the carbon produced passes through SCWO).
The main system for oxygen generation onboard DL4
will be from agricultural and recreational plants. For agricultural reasons
there will already be, at any one time, four overall growing ‘seasons’ for agricultural
plants. These will relate roughly to spring, summer, autumn and winter, allowing
for the year round production of all foodstuffs. The ‘seasons’ will be further
divided into climates, however this has been dealt with in more detail in 9.3
Agriculture Area Layout. For life support reasons, the four ‘season’ areas
will be doubled, thus creating two springs, two summers, and so on. One out
of every two of these will approximate daytime conditions, and thus the production
of oxygen, while the other will approximate night-time conditions, and thus
the production of carbon dioxide (see fig. 17.12a). Plants in the recreational
area will grow according to the seasons within the human habitat. These can
more or less be discounted from oxygen generation as they will be, in the main,
fully grown trees (eventually), grass, and flowering plants, all of which are
less effective at decreasing carbon dioxide levels in the atmosphere.
Therefore, in terms of oxygen generation, the main
plants, at any one time, to be considered will be the agricultural daytime plants
undergoing their spring season. These plants, in the height of their photosynthesis
year, will be capable of producing 0.85kg of oxygen for every 1.12kg carbon
dioxide fed to them. This 0.85kg of oxygen supplies the needs of one person
for one day, however the average person only produces 1kg of carbon dioxide
per day (ref. 18). This gap between supply and demand should however
be overcome due to other sources of CO2 onboard DaedalusaL4, such
as the ‘night-time’ plants, SCWO, the recreational plants, biological waste
decomposition and VAPCAR. Thus, assuming the extra CO2 needs are
met and not exceeded an agricultural plant growing area of 217,300m2
(including non-food products grown in the agricultural section – cotton, flax)
will be capable of recycling the necessary oxygen for 10,000 residents back
into the atmosphere. However, there are other sinks for oxygen onboard DaedalusaL4,
such as SCWO, VAPCAR, TCC (Trace Contaminant Control – Reactive Bed Plasma,
see 17.10), Biological Nitrification and Biological Waste Decomposition, along
with others. The addition of 651,900m2 for those plants that are
out of season (not at the height of their photosynthesis year) will easily meet
these needs with the help of the ‘recreational plants’. Assuming a total population
presence and allowing a generous buffer zone to compensate for any failed crops,
this brings the agricultural area up to 869,200m2, although not all
of this may be used at the one time, depending on oxygen consumption, population
and the ‘preference crops’ being grown. Floor space taken up on the main 1g
level will actually be half this figure (» 434,600m2) as the agricultural
section will be double stacked (with about 5-6m between levels in order to incorporate
robotic harvesting technology and tall crops like maize) to maximise space.
Other rejected oxygen generation options were: Static
Feed Electrolysis – required asbestos for the electrodes –, Superoxides – required
expendable chemicals –, and Cryogenic or high Pressure Storage – not an option
in CELSS.
17.8 Carbon Dioxide Concentration
Carbon Dioxide Concentration is necessary in order
to supply a CO2 rich airflow to the plant habitats, which will act
as the principal carbon dioxide sinks. Such methods of concentration will also
ensure that carbon dioxide levels do not exceed safe limits (above 0.4kPa partial
pressure) within the human habitats.
In order to concentrate carbon dioxide, a process
of Electrochemical Depolarisation Concentration (EDC) was chosen. The EDC reacts
hydrogen (from SCWO) and oxygen with carbon dioxide in an electrochemical cell
to produce an anode, CO2 rich, airflow containing a little hydrogen,
and a cathode, low CO2, airflow, which returns to the atmosphere.
The little hydrogen in the anode exhaust can then be used to carry on the process
or to reduce carbon dioxide and produce water for further electrolysis. The
carbon dioxide rich air stream can be used to revitalise the atmosphere of the
daytime plant habitats.
Due to the nature of the hydrogen/oxygen reaction
EDC produces a DC current, as in a fuel cell. This can be used to power other
CELSS functions. The reactions also generate heat, most of which is carried
away by the various air streams, however a coolant water loop is also necessary.
As shown in fig. 17.8a a typical cell consists of two electrodes separated
by an aqueous electrolyte matrix (Cs2CO3).
 The
major problem with EDC is the additional loads on oxygen generation and humidity
control. However, with plants as oxygen generators this subsystem will only
improve their efficiency in such a role and the CHX systems should be able to
cope with the added moisture (providing more potable water for human consumption).
Other options considered included: Molecular Sieves, Solid Amine Water Desorption
(SAWD) and LiOH. Two possible molecular sieves were the 4- and 2- Bed Molecular
Sieves. Both of these were promising in their previous usage, however the beds
required removal for desorption and regeneration in near vacuum conditions,
making direct CO2 supply to the plant habitats difficult. The SAWD
process was ruled out by its consumption of hygiene water and requirement for
replacement Amine Beds. Finally, LiOH was clearly not acceptable, as it is
a non-regenerable process, requiring expendable chemicals.
The
major problem with EDC is the additional loads on oxygen generation and humidity
control. However, with plants as oxygen generators this subsystem will only
improve their efficiency in such a role and the CHX systems should be able to
cope with the added moisture (providing more potable water for human consumption).
Other options considered included: Molecular Sieves, Solid Amine Water Desorption
(SAWD) and LiOH. Two possible molecular sieves were the 4- and 2- Bed Molecular
Sieves. Both of these were promising in their previous usage, however the beds
required removal for desorption and regeneration in near vacuum conditions,
making direct CO2 supply to the plant habitats difficult. The SAWD
process was ruled out by its consumption of hygiene water and requirement for
replacement Amine Beds. Finally, LiOH was clearly not acceptable, as it is
a non-regenerable process, requiring expendable chemicals.
17.9 Carbon Dioxide Removal
Carbon dioxide removal is necessary to maintain
the health of the crew. However, it must be carried out by a means that returns
the carbon to the regeneration loop. A number of methods for doing this have
already been outlined in Oxygen Generation. These were Water Vapour Electrolysis
and subsequent carbon dioxide reduction, Direct CO2 Electrolysis,
and Biological Exchange by Plants. The latter of these three will compose the
principal method for CO2 removal onboard DL4, as detailed previously.
17.10 Trace Contaminant Control and Particulate
Removal
Trace contaminants originate from the metabolism
of residents, the out- and off-gassing of cabin materials (plastics, insulation,
adhesives, paints), thermal degradation, leakage and spills. Residents must
be protected from these in order to prevent hazardous contamination through
airborne biological and chemical compounds. The purpose of TCC (Trace Contaminant
Control) is to do this in a manner that involves the conversion of these contaminants
to harmless gases in non-consumable ways. Acceptable levels of these contaminants
are provided in Atmosphere Monitoring and Control (table 17.11a).
For the purposes of TCC, two main options were considered.
A conceptual design, incorporating a number of current technologies such as
charcoal beds (one regenerable and one non-regenerable), filters, sorbents (such
as LiOH), and catalytic oxidisers, was rejected as it was not compatible with
the ideal of a CELSS (the non-regenerable charcoal bed removed materials from
the loop and other features, such as the sorbents, also required replacements
and re-supply). Instead a Low Temperature Plasma Process consisting of Reactive
Bed Plasma was chosen.
In this system contaminants are oxidised at low
temperatures (394K) using a combination of plasma and catalyst. Process air
is passed through an annular (ring-shaped) reactor where it is partially ionised
into plasma by an alumina catalyst. The air then passes into the main reaction
chamber where plasma-generated high-energy and, subsequently produced, species
(active oxygen) oxidise toxic materials. The Reactive Bed Plasma (RBP) includes
treatment of toxic reaction products (i.e. – nitrous oxides). Luckily the RBP
also acts as an electrostatic precipitator to collect and deactivate particulate
matter.
Despite some problems due to electromagnetic interference
and its high power requirements the RBP system should be capable of acting as
a long-term, low temperature TCC subsystem. Even if problems do arise with
such a new technology the RBP will be operating simultaneously with SCWO, which
deactivates all trace contaminants in the process atmosphere it takes in.
17.11 Atmospheric Monitoring
Atmospheric Monitoring must take place to ensure
that all the above systems for atmosphere regeneration are functioning properly,
especially those dealing with trace contaminant control and carbon dioxide removal.
For this purpose two systems were chosen, in addition to any special sensors
that will be necessary for specific gases, in labs etc.
The first system, called FTIR (Fourier Transform
Infrared) Spectroscopy, involves passing infrared radiation through a sample
of cabin air then creating an IR absorption spectrum with an interferometer.
This signature reveals the constituents of the air sample. The system is unfortunately
incapable of distinguishing between related compounds and does not detect some
compounds, which do not absorb IR radiation, e.g. – those of hydrogen and chlorine.
Due to these disadvantages this system will be used in conjunction with an Ion
Trap Mass Spectroscopy/Mass Spectroscopy (MS/MS). This fills in what gaps FTIR
leaves but is incapable of distinguishing substances of identical molecular
weight.
Once in the ion trap of MS/MS the cabin air sample
is ionised before being separated on the basis of molecular weight and analysed
by an ion detector. The more current Gas Chromatograph leading to Mass Spectrometer
(GC/MS) system was rejected as it requires trace contaminant concentration before
analysis whereas MS/MS offers the same detailed information in the fastest time.
This system along with Trace Contaminant Control must ensure that trace contaminant
levels are maintained below the safety levels recommended aboard the Space Shuttle
(see table 17.11a).
The atmospheric composition of the main gases (CO2,
O2, N2) will vary across the settlement depending on the
habitat (i.e. – slightly higher carbon dioxide levels in plant habitats to maximise
photosynthesis). However, in the main human habitat, the atmosphere will closely
resemble that found at sea level on Earth. Therefore, a partial pressure of
22.7kPa of oxygen will be maintained to ensure proper alveolar functions and
to prevent losses in blood cell mass or the growth of bacterial epidemics.
Carbon dioxide should be kept at a partial pressure below 0.4kPa in order to
ensure the safety of the crew and maximise human habitat plant photosynthesis.
The idea of a decreased partial pressure of nitrogen was rejected on the basis
that this would lower the atmosphere’s resistance to fire and that the overall
reduction in atmospheric pressure would cause a safety hazard when it came to
operating some of the high pressure systems onboard (i.e. – SCWO and VAPCAR).
Therefore, a partial pressure of 53.2kPa was chosen for nitrogen. This unfortunately
must be made up initially from terrestrial sources as we are not aware of any
significant nitrogen sources on asteroids or the Moon.
|
Trace Contaminant
|
Molar Weight
(for MS/MS detection)
|
Maximum Allowable Concentration (mg/m3)
|
|
Alcohols
|
32
|
10
|
|
Aldehyds
|
56
|
0.1
|
|
Aromatic Carbohydrates
|
78
|
3
|
|
Esters
|
102
|
30
|
|
Ethers
|
68
|
3
|
|
Chloridcarbons
|
93
|
0.2
|
|
Chloridfluorcarbons
|
68
|
24
|
|
Cluorocarbons
|
70
|
12
|
|
Carbohydrates
|
72
|
3
|
|
Inorganic Acids
|
20
|
0.08
|
|
Ketons
|
142
|
29
|
|
Merkaptans
|
48
|
2
|
|
Nitrogen oxides
|
46
|
0.9
|
|
Organic Acids
|
60
|
5
|
|
Organic Nitrogens
|
46
|
0.03
|
|
Organic Sulphides
|
90
|
0.37
|
|
Ammonia
|
17
|
17
|
|
Carbon monoxide
|
28
|
17
|
|
Cyanhydrogen
|
27
|
1
|
table 17.11a – Safe Trace Contaminant Levels
(US Space Shuttle)
DL4’s atmospheric oxygen supply will be harvested
from lunar soils. This process involves the crushing and screening of the ore,
the processing of the ore and the liquefaction and integration of oxygen into
DL4’s atmosphere (ref. 35). Tailings produced, given a mere 10% ilmenite
– FeTiO3 – (the compound from which water will be initially removed
through reduction with hydrogen[3], and then from which oxygen will
be harvested by electrolysis, ref. 19) recovery rate from the soil, allows
for such surplus materials to then be stored and processed for industrial metal
extraction and for the creation of radiation shielding. Therefore, as all mined
lunar soil will be put to use on DaedalusaL4 and given the settlement’s receipt
of almost constant sunlight, it makes sense to perform lunar oxygen extraction
directly from regolith (collected on the Moon) onboard the settlement during
its construction phase. The need for a constant power source arises as the
power-up and -down stages of the harvesting process lower the efficiency of
the system to around 45% thus possibly requiring nuclear power to make the process
viable. Constant sunlight will also provide for a solar furnace (although some
problems have been envisaged due to mirror and window contamination). The processes
carried out onboard DL4 can be easily automated or tele-operated and thus the
production of oxygen for the settlement, as it is being built, should not increase
the work force greatly.

(Click to enlarge)
17.12 Conclusion
Success in the closure of the various subsystems
of Life Support is measured in K values, K representing the closure
itself. For every substance recycled:

where E is the rate of consumption in the
system, e is the rate of (incomplete) closure, and I is a constant
(ref. 30). The goal, K = I – 1, is unfortunately unreachable,
but asymptotically approaching it will be every biologists’ favourite game on
DL4 and critical to achieving a self-sustaining existence. The proposed subsystems
above along with our agricultural proposals will hopefully provide the maximum
closure possible to attain this dream.

 VAPCAR produces
near potable water, which only requires slight alteration of its pH to meet
the necessary standards (detailed later in 17.5 Water Monitoring). Therefore,
the water produced can either be put into plant circulation for further purification
or placed after being tested in potable water storage for human consumption,
depending on the needs at the time (see fig. 17.12a). The remaining
outputs are mostly gases that can go directly into the atmosphere. Some problems
with the compressor system within VAPCAR have arisen, however these should be
solved shortly and given the futuristic nature of this project are not seen
as posing any significant difficulties.
VAPCAR produces
near potable water, which only requires slight alteration of its pH to meet
the necessary standards (detailed later in 17.5 Water Monitoring). Therefore,
the water produced can either be put into plant circulation for further purification
or placed after being tested in potable water storage for human consumption,
depending on the needs at the time (see fig. 17.12a). The remaining
outputs are mostly gases that can go directly into the atmosphere. Some problems
with the compressor system within VAPCAR have arisen, however these should be
solved shortly and given the futuristic nature of this project are not seen
as posing any significant difficulties.
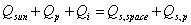
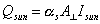
 In
order to ensure these parameters are met, the pH, ammonia content, total organic
carbon (TOC), electrical conductivity and microbial concentration must be frequently
monitored. Microbial content can be monitored indirectly by measuring the iodine
content of the water, thus ensuring the constant presence of a biocide. A suitable
procedure for water quality monitoring is given in fig. 17.5a. This
could easily be implemented before water produced from CHX, VAPCAR and SCWO
subsystems are inputted into water storage.
In
order to ensure these parameters are met, the pH, ammonia content, total organic
carbon (TOC), electrical conductivity and microbial concentration must be frequently
monitored. Microbial content can be monitored indirectly by measuring the iodine
content of the water, thus ensuring the constant presence of a biocide. A suitable
procedure for water quality monitoring is given in fig. 17.5a. This
could easily be implemented before water produced from CHX, VAPCAR and SCWO
subsystems are inputted into water storage.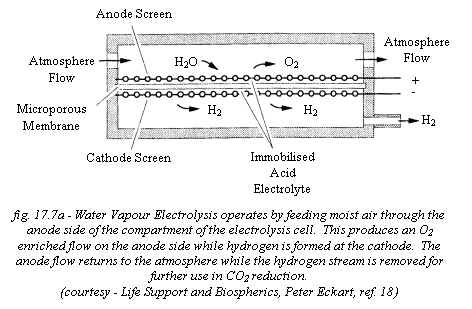
 The
major problem with EDC is the additional loads on oxygen generation and humidity
control. However, with plants as oxygen generators this subsystem will only
improve their efficiency in such a role and the CHX systems should be able to
cope with the added moisture (providing more potable water for human consumption).
Other options considered included: Molecular Sieves, Solid Amine Water Desorption
(SAWD) and LiOH. Two possible molecular sieves were the 4- and 2- Bed Molecular
Sieves. Both of these were promising in their previous usage, however the beds
required removal for desorption and regeneration in near vacuum conditions,
making direct CO2 supply to the plant habitats difficult. The SAWD
process was ruled out by its consumption of hygiene water and requirement for
replacement Amine Beds. Finally, LiOH was clearly not acceptable, as it is
a non-regenerable process, requiring expendable chemicals.
The
major problem with EDC is the additional loads on oxygen generation and humidity
control. However, with plants as oxygen generators this subsystem will only
improve their efficiency in such a role and the CHX systems should be able to
cope with the added moisture (providing more potable water for human consumption).
Other options considered included: Molecular Sieves, Solid Amine Water Desorption
(SAWD) and LiOH. Two possible molecular sieves were the 4- and 2- Bed Molecular
Sieves. Both of these were promising in their previous usage, however the beds
required removal for desorption and regeneration in near vacuum conditions,
making direct CO2 supply to the plant habitats difficult. The SAWD
process was ruled out by its consumption of hygiene water and requirement for
replacement Amine Beds. Finally, LiOH was clearly not acceptable, as it is
a non-regenerable process, requiring expendable chemicals.